
Blood Gas Analysis is also called Arterial Blood Gas Analysis (ABG), which measures the amount of Oxygen & Carbon dioxide in the blood and also measures the pH of the blood.
Blood gas analysis is performed on blood collected from the artery. It measures the partial pressure of Oxygen and Carbon dioxide in the blood, as well as Oxygen content, Oxygen saturation, Bicarbonate content and Blood pH.
Oxygen in the lungs is carried to the tissues through the bloodstream, but only a small fraction of this oxygen dissolves in the arterial blood. How much dissolving depends on the partial pressure of oxygen, therefore, testing the partial pressure of Oxygen indicates how much Oxygen the lungs deliver to the blood. Carbon dioxide is released into the blood as a by-product of cell metabolism. Partial Carbon dioxide pressure indicates how well the lungs are removing this Carbon dioxide.
The rest of the insoluble Oxygen in the blood is mixed with Hemoglobin, a Protein - Iron compound. The Oxygen content measurement in the ABG Analysis indicates how much Oxygen is mixed with Hemoglobin.
Purpose of carrying out Blood Gas Analysis
- Blood Gas Analysis is usually done to assess respiratory illnesses and other conditions that can affect the lungs and to manage patients receiving Oxygen therapy.
- The ABG test is performed to evaluate how efficiently the lungs supply Oxygen to the blood and how effectively it removes carbon dioxide from the blood.
- The Test also shows how the Lungs and Kidneys work together to maintain normal Blood pH levels (Acid-Base Balance).
- In addition, the Acid-Base component of the test provides insights into the condition of kidney function.
An imbalance in the Oxygen, carbon dioxide and pH levels of your blood can be an indication of certain medical conditions.
These may include:
- Kidney failure
- Heart failure
- Uncontrolled diabetes
- Chemical poisoning
- Hemorrhage
- Drug overdose
- Shock
When you exhibit symptoms of any of these conditions, your doctor may order a blood gas test. This test requires a small amount of blood to be collected from an artery. The process is safe and simple, and it only takes a few minutes to accomplish.
Why Blood Gas Test is Done?
The levels of oxygen and carbon dioxide in the body can be accurately measured with a
blood gas test. By doing so, you can help your doctor determine how healthy your lungs and kidneys are.
Tests such as these are commonly used in hospital settings to determine the treatment for acutely ill patients. In the primary care setting, it may not play a very significant role, but it can be used in a pulmonary function lab or clinic.
A Blood Gas Test may be ordered by your doctor if you are displaying symptoms of oxygen deficiency, carbon dioxide accumulation or acid reflux.
Some of the Symptoms may Include:
- Shortness of breath
- Difficulty breathing
- Confusion
- Nausea
These symptoms are often associated with conditions such as Asthma and Chronic Obstructive Pulmonary Disease (COPD).
Blood Gas Tests are also ordered if your doctor suspects you have a medical condition such as:
- Lung disease
- Kidney disease
- Metabolic disease
- Head or neck injuries that affect breathing
Your blood’s pH and gas levels can also provide your doctor with useful information for monitoring treatment for certain conditions, such as lung and kidney disease. In addition to blood gas testing, a blood glucose test is often ordered to check Blood Sugar levels and a Creatinine Test to monitor Kidney function.
Results Interpretation
The results of a Blood Gas Test are used by your doctor to diagnose various diseases and diagnose whether treatment is working for certain ailments, such as lung disease. The test also determines whether your body compensates for the imbalance.
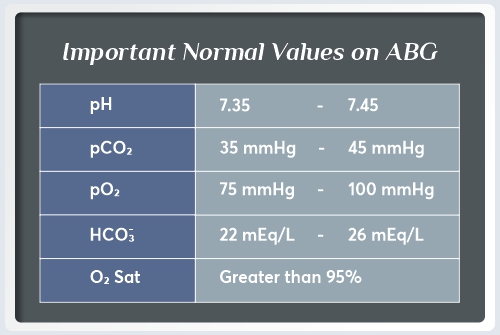
Arterial Blood pH: Normal range: (7.35 to 7.45)
The Arterial Blood pH measures the number of hydrogen ions in the blood. Generally, pH levels under 7.35 are referred to as acidic, and pH levels over 7.35 are referred to as basic, or alkaline. Having a lower blood pH could be an indication that your blood is more acidic and that there is more carbon dioxide in your system. High blood pH indicates highly alkaline blood with high levels of bicarbonate in your body.
Bicarbonate: Normal Range: (22 to 26 mEq/ L)
Bicarbonate supports the regulation of the pH of the blood by keeping things from becoming too acidic or too basic.
The Partial Pressure of Oxygen: Normal Range : (75 to 100 mmHg)
The Partial Pressure of Oxygen in the blood measures how much oxygen is dissolved in it. In other words, this measures how well oxygen enters the bloodstream from the lungs.
The Partial Pressure of Carbon Dioxide: Normal Range: ( 35 to 45 mmHg)
An indicator of the amount of Carbon dioxide contained in the blood is called partial pressure of carbon dioxide. i.e The ability of the body to expel carbon dioxide is determined by this parameter.
Oxygen Saturation: Normal Range: ( >95% )
A measurement of how much oxygen is carried by the hemoglobin in red blood cells is oxygen saturation.
If the values are derived from capillary or venous samples, the reference range for the normal values will be slightly different.
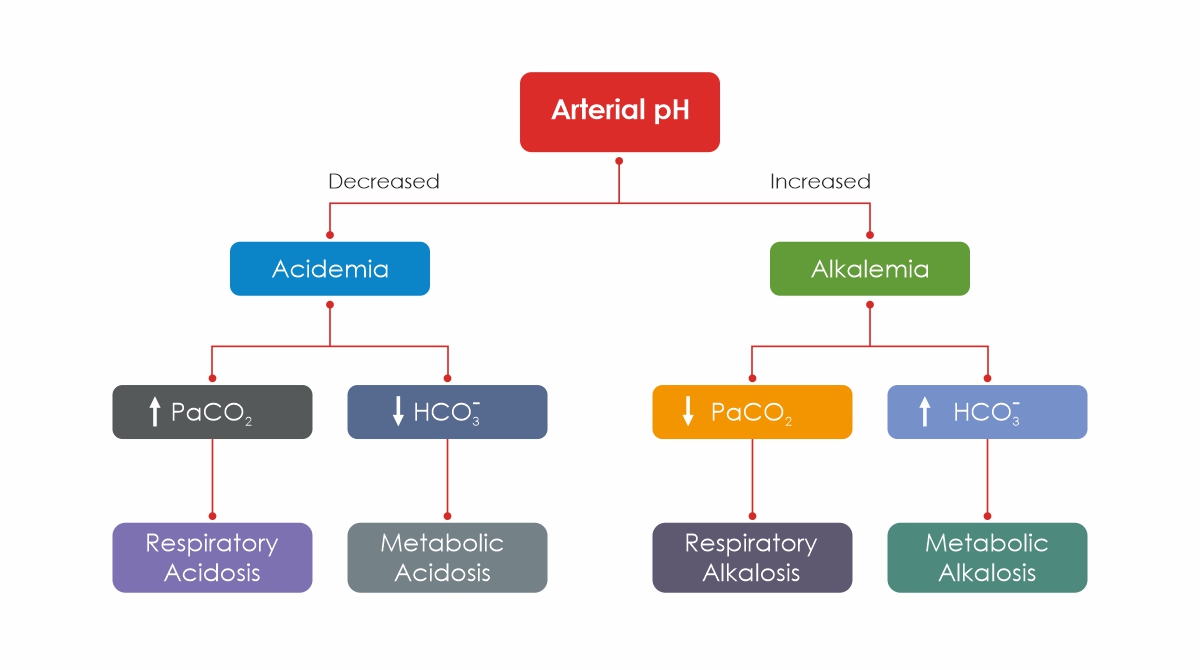
Some Medical Conditions, including those listed below, can cause Abnormal Results:
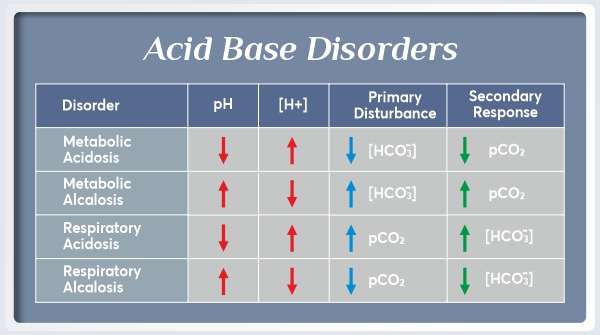
Condition 1: Metabolic Acidosis
An electrolyte imbalance in the body is the basis of metabolic acidosis, which is a serious electrolyte disorder. There are three major causes of Metabolic Acidosis: Excessive acid production, Bicarbonate loss, and a reduced ability to remove excess acid from the body.
Common Causes: Shock, Kidney failure Diabetic Ketoacidosis
Condition 2: Metabolic Alkalosis
The Alkalosis of the metabolism is a condition in which the tissues have a pH that is above normal (7.35–7.45). It results from reduced hydrogen ion concentration, which in turn causes an increase in bicarbonate, or else it is a direct consequence of an increase in bicarbonate concentrations.
Common Causes: Low Blood potassium, Chronic Vomiting
Condition 3: Respiratory Acidosis
As a result of Respiratory Acidosis, the lungs are unable to remove all of the carbon dioxide produced by the body. In turn, body fluids become excessively acidic, especially blood.
Common Causes: Including Pneumonia or COPD, Lung diseases
Condition 4: Respiratory Alkalosis
A medical condition involving elevated blood pH and a reduction in arterial carbon dioxide levels, respiratory Alkalosis occurs when increased respiration elevates blood pH beyond the normal range (7.35–7.45).
Common Causes: Pain, Breathing too fast or Anxiety
|
Acid Base Disorder PH Value
|
|
Acid Base Disorder
|
PH Value
|
PCO2
|
|
Metabolic Acidosis
|
<7.35
|
Low
|
|
Metabolic Alkalosis
|
>7.45
|
High
|
|
Respiratory Acidosis
|
<7.35
|
High
|
|
Respiratory Alkalosis
|
>7.45
|
Low
|















